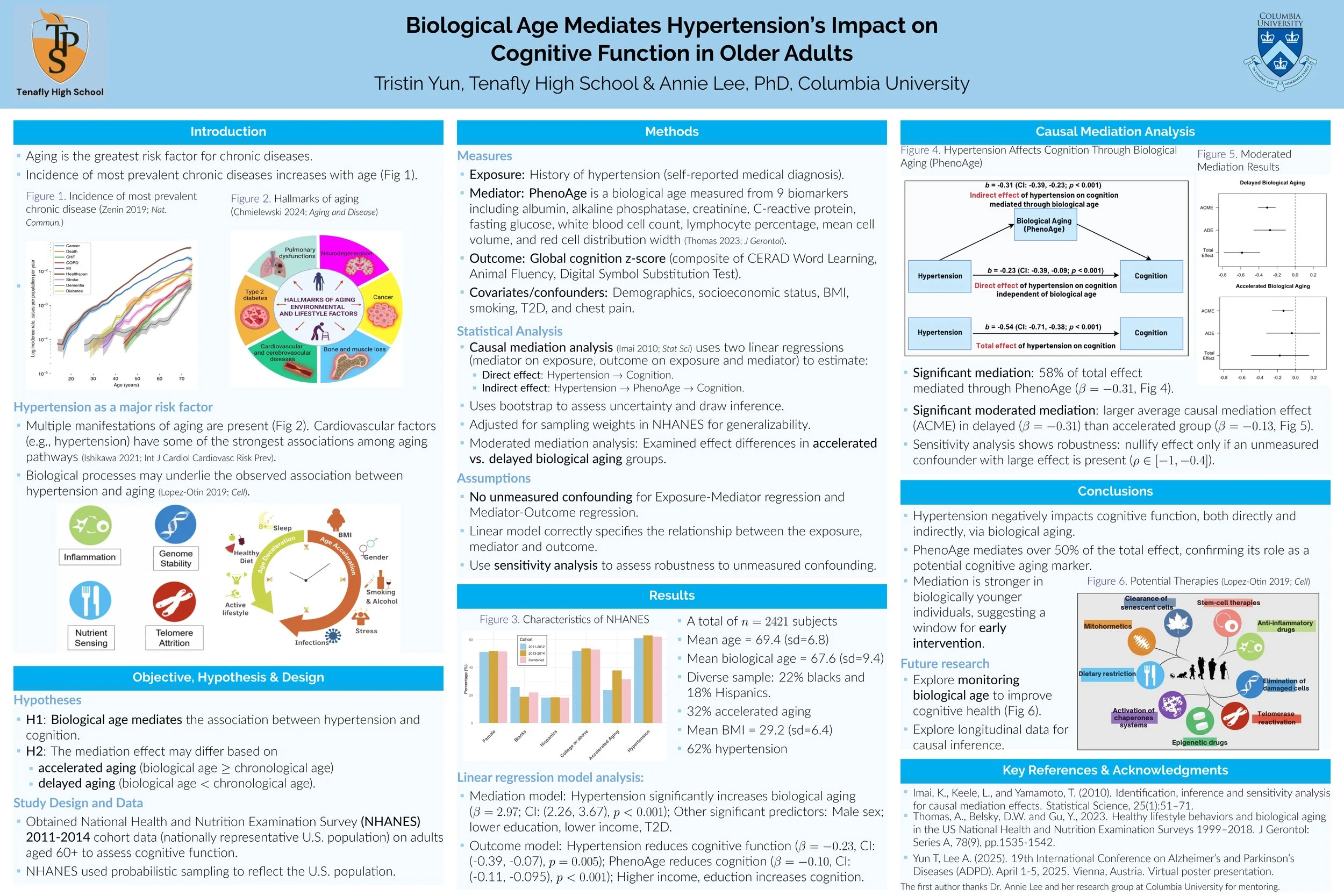
My ADPD experience.
Last month, I had the opportunity to present a virtual poster at ADPD 2025, the premier global conference focused on Alzheimer’s disease (AD), Parkinson’s disease (PD), and other neurodegenerative disorders, including Huntington’s disease (HD). My poster builds on research I began in the summer of 2024, investigating biological aging as a pathway linking cardiovascular risk factors to cognitive decline in older adults. You can view our e-poster and listen to the accompanying audio presentation here.
What made this experience especially exciting was discovering a direct connection between my research and emerging therapies in AD drug development.
The urgency is clear: the Alzheimer’s Association projects that 13.8 million Americans will be living with AD by 2050, with global cases expected to soar to 152 million. But for the first time in decades, the field is feeling a real sense of momentum.
One of the most significant breakthroughs is the FDA’s recent approval of lecanemab, a monoclonal antibody targeting amyloid-β, the protein believed to play a key role in AD pathology. Unlike past treatments that only addressed symptoms, lecanemab aims to slow disease progression by reducing amyloid plaque buildup. As shown in the figure below, traditional symptomatic treatments offer temporary cognitive improvements without altering the disease’s underlying biology. In contrast, disease-modifying therapies like lecanemab alter the trajectory of decline, delaying progression by targeting root causes.
Another drug on the horizon is donanemab, which is currently under FDA review. Like lecanemab, donanemab is an anti-amyloid antibody, but with a crucial difference: while lecanemab has shown effects on biomarkers (amyloid-β levels), donanemab has demonstrated clinical improvement in cognitive outcomes. This marks a vital step forward in patient-centered care.
Beyond anti-amyloid strategies, there’s growing interest in GLP-1 receptor agonists, a class of drugs originally developed for diabetes, that may offer neuroprotective benefits. These therapies appear to modulate metabolic pathways linked to cognitive decline. Intriguingly, this aligns with my own research into biological aging and metabolic dysregulation as mechanisms underlying Alzheimer’s.
The science is converging in powerful ways. From basic biology to translational therapeutics, we're witnessing a shift from managing symptoms to targeting the root drivers of neurodegeneration.
ADPD 2025 left me hopeful. The pace of innovation is accelerating, and by the time my generation may face cognitive aging, I believe we’ll have multiple effective therapies available, not just to treat, but to prevent and delay Alzheimer’s disease.